
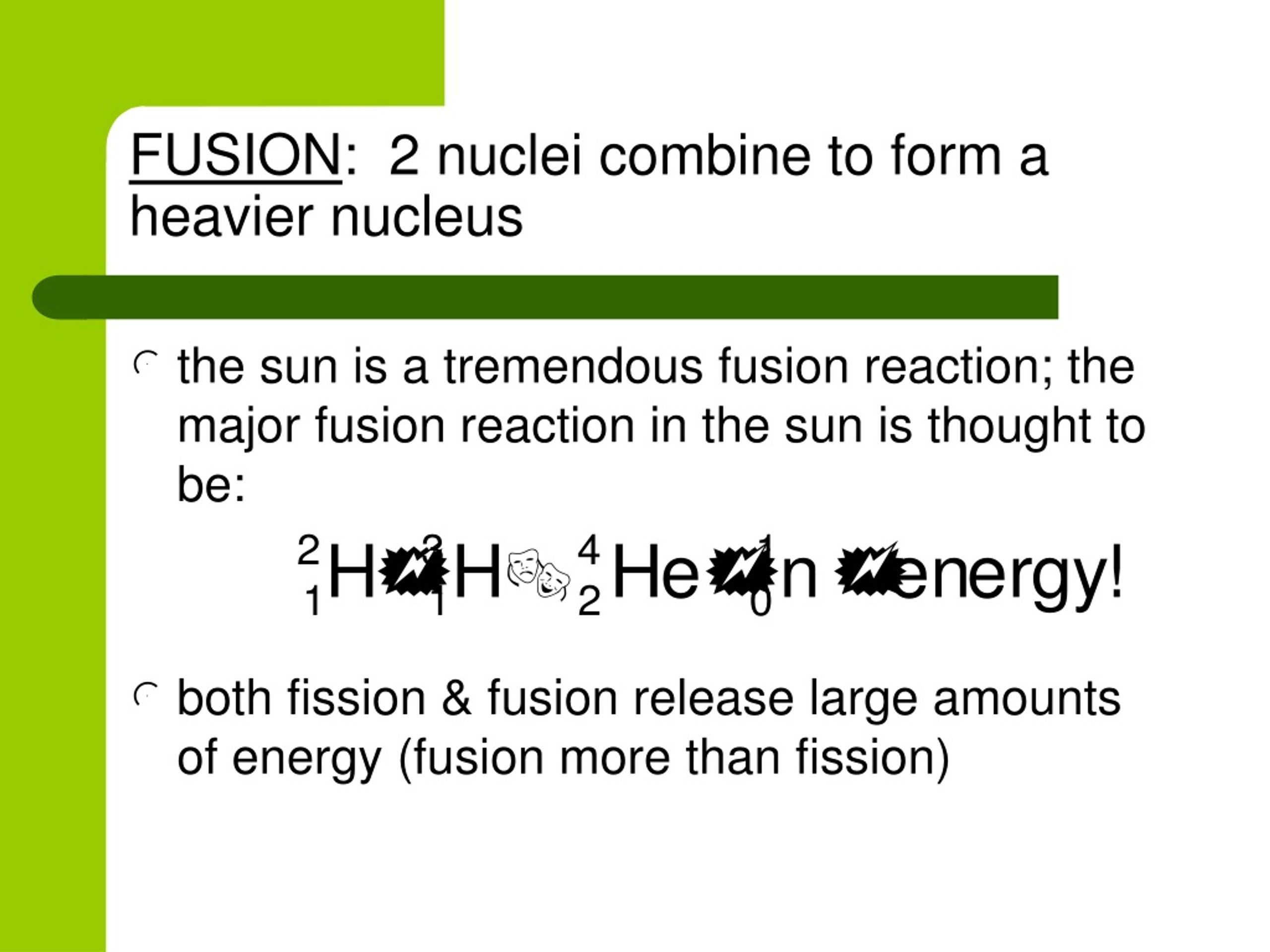
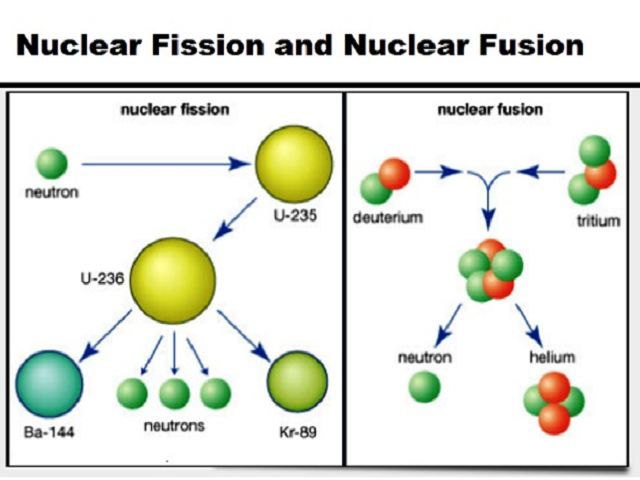
The nuclide notation can be represented as MEAN which stands for Visualization can be very helpful in understanding difficult topics like nuclear fusion, there are few options other than using mnemonics. Discover Labster's Fusion virtual lab today! 4. If scientists find a way to harness fusion energy in machines on Earth, it could become an important method for generating electricity. Einstein's equation (E=mc2), which states that mass and energy can be converted into each other, explains why this process occurs. The remaining mass is converted into energy. This process releases energy because the total mass of the resulting single nucleus is less than the mass of the original two nuclei. In a fusion reaction, two lighter nuclei join together to form a heavier nucleus. The Sun and other stars are powered by nuclear fusion. However, it can be altered by weak interactions such as beta decay, which can occur with radioactivity and nuclear fusion where atomic number is not conserved. The atomic number is only conserved in most cases, for example in chemical reactions and nuclear fission. The mass number is conserved in all interactions, including chemical reactions, radioactive decay, fusion, and fission. In physics, a quantity is conserved if it is the same before and after the interaction. The law of conservation of nuclear physics Relate it to the real worldįigure 7: A snippet from the nuclear fusion simulation Labster. This understanding of nuclear fusion is linked to Hans Bethe's work on stellar nucleosynthesis, in which he describes how the sun and stars release energy through proton-proton chain reactions.

This is the first live demonstration of fusion in the laboratory. His student Mark Oliphant used an updated version of the apparatus, firing deuterium instead of hydrogen and discovering helium-3 and tritium, showing that heavy hydrogen nuclei can be tricked into reacting with each other. In his famous 1934 experiment, Rutherford demonstrated the fusion of deuterium into helium and found that a "major effect" was produced during the process. And at the same time, Ernest Rutherford was studying the structure of the atom. Eddington's theory was first published in 1926 in The Internal Structure of the Stars and laid the foundation for modern theoretical astrophysics.įollowing Eddington's article, Robert d'Escort Atkinson and Fritz Hautermans gave the first calculations of the rate of nuclear fusion in stars. However, the science and physics of nuclear fusion did not become clear until the 1920s, when British astrophysicist Arthur Eddington proposed that stars derive their energy from hydrogen fusion into helium. Nuclear fusion is the most dominant reaction in our observable universe, and it is this reaction that drives our sun and stars. With these points in mind, here are five things you can add to nuclear fusion class to make it more engaging, accessible, and fun for you and your students.
In this case, we can call the nuclide 32He 'helium three'.įigure 3: A nucleus exhibits strong nuclear attraction (yellow) and electrostatic repulsion (red). An element always has the same number of protons, so the atomic number is sometimes omitted. The element symbol indicates the element's name (e.g. When we subtract the atomic number from the mass number, the number of neutrons is gotten.
The atomic number is the number of protons, it is indicated in the index to the left of the element symbol. This is indicated in the superscript to the left of the item icon. The mass number is the total number of nucleons (protons and neutrons). Nuclide notation: The number of protons and neutrons in a nucleus is represented by nuclide notation. Not being able to visualize the process and not seeing its relevance to the real world can demoralize learning and make it difficult for students to stay motivated. Feels abstractīecause fusion occurs at the subatomic level, you can't see it. There are three reasons why nuclear fusion can be difficult for even the most active students.

Figure 1: A snippet from the nuclear fusion simulation Labster.


 0 kommentar(er)
0 kommentar(er)
